Stem Cells by Blue Horizon
Our research
Our research has been published in “Experimental and therapeutic medicine” cited at PubMed Central, US National Library of Medicine, National Institutes of Health; “CellR4”, the official journal of The Cure Alliance...
Our Stem Cell
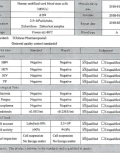
Our stem cell products evaluated in clinical studies are collected in accordance with Good Tissue Practice to ensure safety. The studies have been conducted following approval by an Investigational Research Board...
Our Results
Our results show our cellular products are safe and effective in the improvement of symptoms related to chronic inflammation, spinal cord injury, stroke, musculoskeletal disorders and other medical conditions.
Cultured Wharton’s Jelly And Umbilical Cord Blood
Cultured Wharton's Jelly, along with stem cell therapies derived from umbilical cord blood and adipose tissue, are showing promise in clinical trials for treating various medical conditions, such as osteoarthritis, spinal cord injury, and heart disease.
acute ischemic stroke
The Food and Drug Administration (FDA) has given us approval to proceed with our Investigational New Drug (IND) application allowing for the initiation of a clinical study of an FDA-approved cell therapy (HCP, cord blood)...